The Challenges of Labeling for OSHA's Revised Hazard Communication Standard
As a response to the multiple definitions of "hazard" and multiple ways of communicating hazards, the United Nations adopted the Globally Harmonized System for Classification and Labeling of Chemicals (GHS) in 2003.
OSHA's revised Hazard Communication Standard has presented manufacturers, formulators and distributors with the challenge of revising their safety data sheets (SDSs) and the product labels by June 1. These changes are based upon the third revision of the GHS.
The challenges to implementing GHS include the mandatory use of red color, the potential need for multiple languages if shipping to other countries, various U.S. state issues like New Jersey's "Right to Know" that go beyond OSHA's requirements and many other regional regulatory requirements for compliance in the global marketplace. The reality is that virtually every label for a hazardous chemical product is subject to change, and will in many cases require changes on an ongoing basis in the unforeseeable future.
Complicating the environment in which these regulations will go into effect, the chemical industry is faced with the major challenge due that many large companies have decentralized their hazard communication work processes. In addition, many medium- to smaller-sized companies don't have the internal resources to create their own safety data sheets and must use outside resources. Because of the additional requirements in the 2012 OSHA and GHS regulations to be implemented starting June 1, regardless of how or where a safety data sheet is created, automated systems will need to be capable of pulling the information from Section 2 of the SDS onto labels. In addition, the current, complex nuances of labeling range from having many different products of various shapes and sizes, customer requirements, accessing transactional data, multiple languages, branding information and more.
Why the GHS?
Before adoption of the GHS, multiple systems and definitions of hazard were the rule. Even here in the United States there have been – and to some extent still are – different definitions of various physical and health hazards presented by chemical substances. Looking at just two hazards, flammability and oral toxicity, shows the disparity in definitions, and how the GHS has created a common basis for these two frequently encountered hazards. These hazards, as shown in the charts on this page, were compared based on 2009 regulations, because many countries already have adopted, or are in the process of adopting, GHS definitions.
For instance, the European Union adopted GHS for substances in 2010 and the classification and labeling of mixtures is scheduled to become mandatory by June 1, which is the same day as OSHA's mandatory implementation date. Canada actively is working on the institution of GHS but will not be able to complete implementation for industrial products by 2015. Accordingly, they are trying for mandatory implementation by manufacturers by June 1, 2016, and a complete implementation by June 1, 2017, when stock on shelves no longer can be shipped with older formatted labels. Therefore, between June 1, 2015 and June 1, 2016, shippers in the United States may need to create a separate label for Canadian shipments.
This difference in implementation timelines is an example of why a single product might need two different labels depending on its final destination. For the time being, industrial and consumer labels in the United States and Canada will continue to differ, while by next year, European industrial and consumer labels will follow the same classification and communication scheme.
Oral toxicity is even more complicated, and could be the subject of its own article regarding all the conflicting definitions that existed in 2009, but here is the unified definition developed under GHS: There are different GHS tables for dermal toxicity and three for inhalation, one each for gases, vapors and dusts and mists. All would have to be examined in creating a new label.
Also, the various shapes of symbols and graphics used for hazard communications are being unified into a single shape and graphic that will be used for both transport and for workplace notification. This will require a change for all EU labels for mixtures – both industrial and consumer – beginning June 1, and will change Canadian industrial labels by June 1, 2016. The new graphic will be mandatory.
Some Things Don't Change
Labels will now have more information on them, and will have to be revised to include symbols, standard signal words and standard phrases. Other text, such as contact phone numbers and statements about ingredients with unknown toxicity also will be required.
And Some Things Change a Lot…
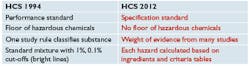
Before 2015, as a performance standard, manufacturers could meet the OSHA requirements by methods of their own choosing. Now as a specification standard, manufacturers must follow methods of compliance outlined by OSHA.
OSHA used to talk about flammability, pressure, explosively and reactivity. It now is more finely defined by GHS into these categories:
➤ Explosives
➤ Flammable gases
➤ Oxidizing gases
➤ Pressurized gases
➤ Compressed gases
➤ Liquefied gases
➤ Refrigerated liquefied gases
➤ Dissolved gases
➤ Flammable liquids
➤ Flammable solids
– Self-reactive substances
– Pyrophoric liquids
– Pyrophoric solids
– Self-heating substances
– Water Reactive producing flammable gases
– Oxidizing liquids
– Oxidizing solids
– Organic peroxides
– Corrosive to metals
– Explosive dusts
OSHA regulates all these hazards and others, like "explosive dusts."
Likewise, health hazards have been more finely defined, but the change is not as dramatic as with physical hazards. The increased number of physical hazards is more in line with worldwide definitions already existing for the transport of dangerous goods.
The changes to health hazards had to accommodate the various international systems with the guiding principle that no country would reduce the level of protection that previously existed. This will impact both safety data sheets and labels. The older definitions of health hazards include:
➤ Irritants
➤ Corrosives
➤ Toxins
➤ Sensitizers
➤ Effects on target organs (i.e. liver, kidney, nervous system, blood, lungs, mucous membranes, reproductive system, skin, eyes, etc.)
The newer definitions include:
➤ Acute toxicity, oral
➤ Acute toxicity, dermal
➤ Acute toxicity, inhalation
➤ Aspiration hazard
➤ Skin corrosion/irritation
➤ Eye corrosion/irritation
➤ Respiratory sensitization
➤ Skin sensitization
➤ Germ cell mutagenicity
➤ Carcinogenicity
– Reproductive toxicity, fertility
– Reproductive toxicity, development
– Specific target organ toxicity (STOT)
– Single Dose
– Repeat Dose
Most of these categories previously were regulated, but now all categories of these hazards are being regulated. It is important to clarify that OSHA will not regulate materials of lower toxicity that would be in the home where children are present; this is because the Consumer Product Safety Commission regulates consumer labels, and that organization has yet to propose adoption of the GHS system.
Where to Start?
First, manufacturers need to classify their products. If dealing with pure substances, this will be an easier task than if classifying mixtures. But either way, the criteria from the GHS is the rule. Classification will take longer than the old methods, and organizations need to begin this process now. The challenge is to work with accurate data. GHS does not require "testing," but it does require obtaining whatever information is available to accurately assess products.
And, following these considerations, here comes an important issue: Because label content will appear on the SDS, both the SDS and the label need to be deployed together. SDS are documents and can be sent out both in paper or as electronic files, but labels need to be applied to the actual package, which is not as easily accomplished. Key challenges of label production that must be accounted for include accommodating for color printing, dealing with different size products, accommodating multiple languages and transactional data.
As the industry now is well aware, OSHA requires a red border on all symbols used to communicate hazard categories. Black will just not do. For many, using pre-printed label stock with red diamonds has become less practical, as the number of possible variations of pictograms needed varies and also requires manual oversight to make sure the correct label stock is being used.
Package size also is an important consideration in labeling, as chemicals can be transported through supply in containers that vary in size from drums to small vials. The label needs to address both OSHA regulations and the size restrictions of the container, so for small packages it is a challenge to effectively use the limited real estate on a label.
Then, there is the issue of dealing with languages on a label. In the United States, English is mandatory while other languages are optional. For most other countries, e.g. European countries, the label must be produced in that country's language but also may require other languages if you sell and transport in other countries.
Extending the challenge of GHS labeling is a common requirement to apply transactional data such as batch numbers, lot numbers or packing dates. This data, in conjunction with the variables of color, size and language, introduce complexity on the label that make pre-printing labels impractical.
Real-time, data-driven labeling is one of the primary pathways of dealing with these issues to ensure that the correct symbols, languages and transactional data appear on labels of any size or shape. This approach also enables manufacturers to leverage the same regulatory content to ensure that the SDS and label agree with each other. The last thing they want is for the SDS to say one thing, and the label to say something else.
Ongoing regulatory changes in the chemical industry, successful GHS compliance and regional regulatory adherence all require rapid labeling changes to be deployed quickly throughout the organization. The ultimate goal is meeting the requirements presented by the GHS at the same time as dealing with the complexity of labeling hazardous materials to protect all participants in the global supply chain. To achieve this goal, companies must first understand the impact and changes that the GHS necessitates while pursuing an approach that accounts for the unprecedented level of complexity and change required for labeling in the chemical industry.
Daniel Levine, CHMM, is president of Product Safety Solutions, a consulting firm providing services in product hazard communication, TSCA compliance and other regulatory areas affecting both manufacturing activities and finished products. Levine's past positions include director of product safety and integrity for AlliedSignal Inc. and president of the Society for Chemical Hazardous Communication (SCHC). For his extensive experience in chemical industry safety, he received SCHC's Lifetime Achievement Award in 2003 and was elected a Fellow for the Institute of Hazardous Materials Management in 2010. He is a chemical industry expert for Loftware.